BTL EMSCULPT NEO
BTL EMSCULPT NEO
Emsculpt Neo is the latest technology from Emsculpt, a body contouring procedure that burns fat and builds muscle without ever having to hit the gym. Treatments combine radio-frequency (RF) energy and high-intensity focused electromagnetic (HIFEM) technology for added heat and more efficient results that haven’t been seen before. Define that 6-pack, lift that booty, tighten your arms, slim down saddlebags and tone up your legs without even a squat using this revolutionary device.
The skin tightening results you’ll see from your sessions of Emsculpt Neo are achieved by intense muscle contractions that are impossible to match the intensity of in the gym. Increase muscle mass, tone up problems areas and reduce excess fat where efforts at diet and exercise have failed in the past. If you’re looking for Emsculpt in Orange County, our experts at the Med Lounge are here to help you get closer to that body you desire. Say goodbye to stubborn fat and hello to a trimmed-up new you with Emsculpt Neo.
Benefits of BTL EMSCULPT NEO
- Efficient fat-burning
- Core-strengthening
- Zero downtime
- Fast-acting
- Muscle-building
- Fat cells are gone for good
- Improvement over time
- Extremely safe
- Painless procedure
- Short treatments
FREQUENTLY ASKED QUESTIONS
Emsculpt Neo is a body contouring device that uses high-intensity radio-frequency (RF) energy and electromagnetic (HIFEM) technology to work your muscles in a way you couldn’t otherwise.
Those rapid contractions you’ll feel with each session are burning fat cells and improving muscle growth.
That combination of simultaneous RF energy and HIFEM technology comes together to create a perfect storm of fat-burning and muscle growth in targeted areas. The muscles are first warmed up for each session using RF heating, much like an intense warmup before a workout.
Those high temperatures also heat the fat cells in the targeted areas, resulting in apoptosis (cell death). This is a goal of the procedure. You want those cells dead, so they don’t come back.
The HIFEM energy in your treatment works to force powerful muscle contractions in the targeted areas. This level of muscle contraction is impossible in a regular workout, no matter how aggressively you tackle those crunches. The result is an overall increase in the number of muscle fibers and cells and growth in muscle volume.
Yes, Emsculpt Neo really works. In an incredibly efficient use of your time, you’re both burning fat and building muscle. The treatment has also been tested in clinical studies for safety and efficacy, providing patients with proven results without surgery or downtime.
Good candidates for Emsculpt Neo are healthy adults who are looking for a targeted approach to problem areas. Anyone who has not seen progress toning their stomachs, arms, legs, thighs or buttocks could benefit from sessions of this body contouring treatment.
Emsculpt Neo can also support muscle wall strength in anyone who has experienced some separation in the muscles, perhaps due to pregnancy or rapid weight loss. Your provider at the Med Lounge will be able to better plot out a treatment plan for you based on your stated goals and desired results. That may mean a combination of body contouring procedures or additional sessions of Emsculpt Neo to get you on track to where you want to be.
Pregnant women and nursing mothers should not be receiving Emsculpt Neo. Talk to your provider if you have any cardiovascular conditions, implants or are experiencing any pain or injuries to targeted areas before booking a treatment.
Emsculpt Neo body contouring treatments are virtually pain-free. Users report some discomfort and a pulling sensation as the device does its work contracting your muscles in the targeted areas. Your provider can reduce the intensity on the device — or increase the intensity — in your session to manage your comfort levels.
You won’t need any downtime after your session. Like what you’d feel after a workout, some minor soreness is normal and usually goes away within a few days.
The loss of fat cells as a result of your treatment is permanent. Those fat cells don’t return once they’ve died off.
The results you’ll see as far as muscle tone and definition after your Emsculpt Neo sessions will depend on your lifestyle choices post-treatment. While those results are expected to last anywhere between six months to a year, especially if you come in for the four recommended sessions, those results can be extended with a healthy diet and exercise.
Emscupt Neo shouldn’t replace healthy living but a way to target stubborn areas that haven’t responded to traditional weight loss and toning methods.
See the amazing difference in our clients, before and after treatment.
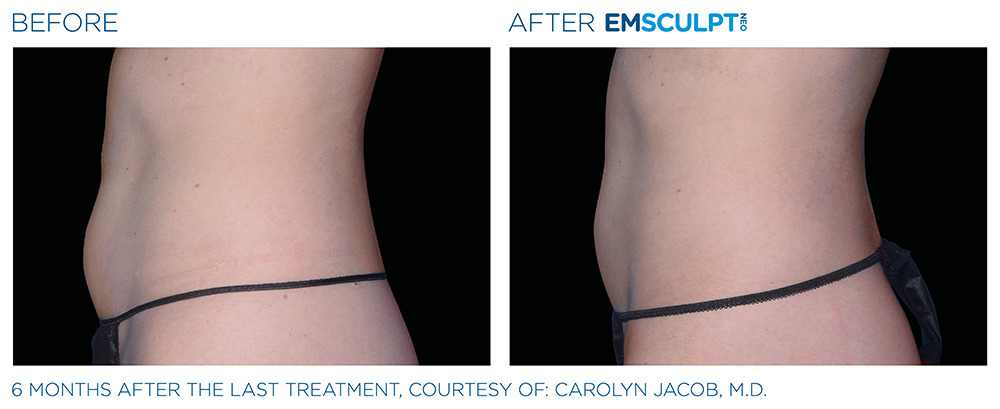
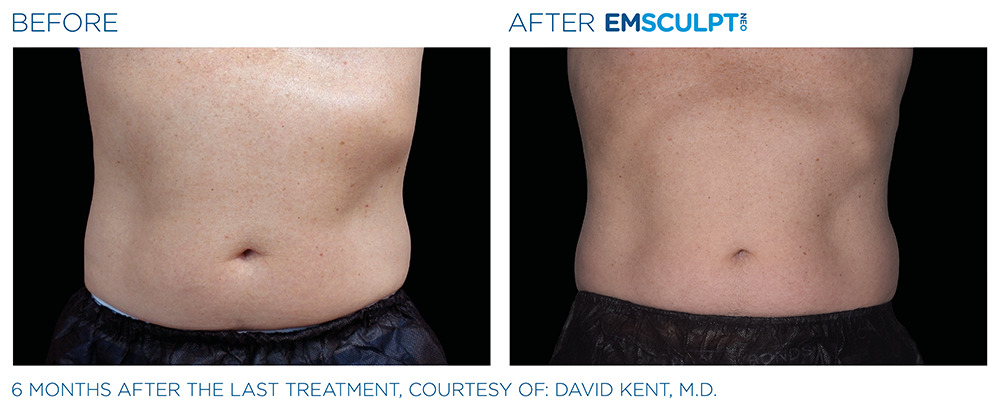
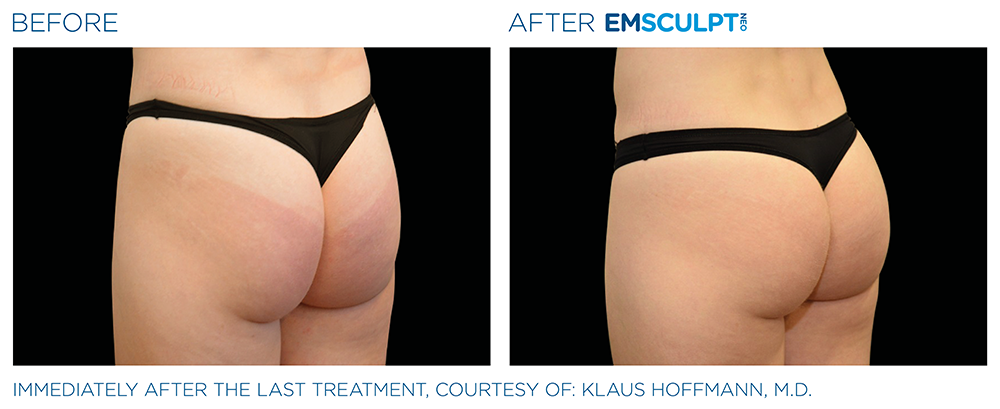
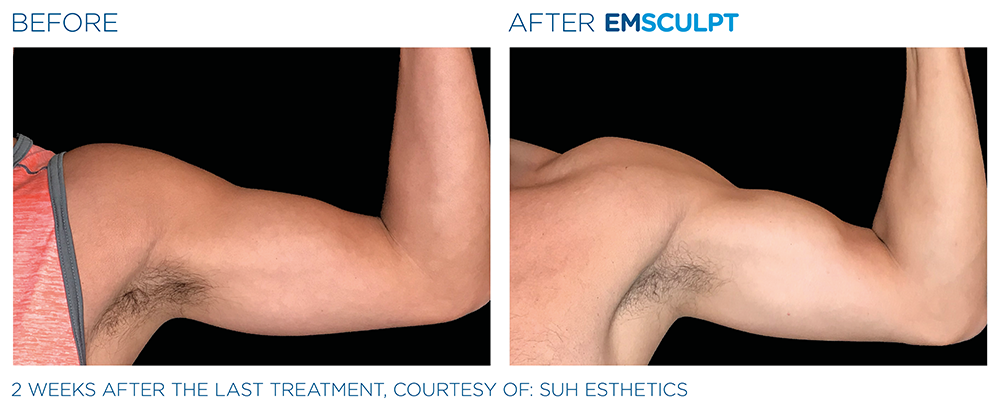
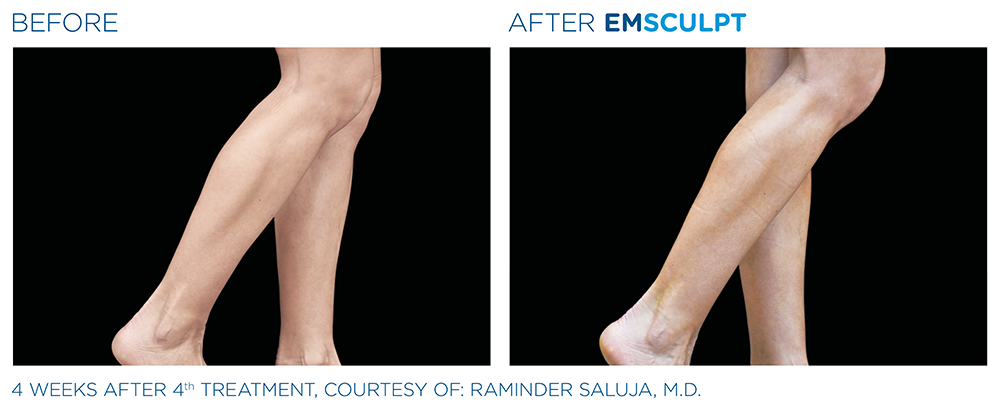
What to Expect With EMSCULPT NEO
Compare the before and after photos of actual patients, to see for yourself why RealSelf users voted the EmsculptNeo “Most Worth It.”
PRE-TREATMENT
- Follow a regular exercise and healthy diet routine before your treatments.
- Drink plenty of water before your appointment so that you aren’t dehydrated. This is essentially an intense workout you’re about to embark on.
- Avoid eating a large meal before your appointment. A light snack is fine, but you don’t want to come to your sessions bloated.
- Wear loose, comfortable clothing to your Emsculpt Neo appointment. Your provider will need easy access to areas being treated by the device.
- Remove any jewelry and put away electronic devices before starting your treatment.
- Talk to your provider about any cardiovascular conditions, implants or injuries you’ve experienced in the areas you’d like to target. Your provider will be able to tell you whether Emsculpt Neo is safe for you or whether you’re a better candidate for a different kind of treatment.
POST-TREATMENT
- There is no expected recovery time after an Emsculpt Neo session. You’ll be able to resume normal activities after your treatment.
- Any tenderness or soreness you feel after a session is normal and should disappear within a few days.
- Listen to your body after your procedure. Drink plenty of water if you’re thirsty and rest if you’re feeling any soreness.
- The recommended number of sessions for optimal results is four sessions over two weeks. The total number of sessions will determine your Emsculpt cost. Schedule your sessions as advised by your provider at the end of your Emsculpt Neo appointment.
- Regular exercise and a healthy eating plan will go a long way in extending your results. Work out those newly toned muscles regularly and maintain a healthy lifestyle to get the biggest bang for your buck with Emsculpt Neo.
- Results and patient experience may vary. Talk to your provider if you’re experiencing any serious symptoms after your sessions, such as intense pain or extreme fatigue.
Safety Information
Emsculpt Neo® is intended for non-invasive lipolysis (breakdown of fat) of the abdomen and thighs and reduction in circumference of the abdomen and thighs with Skin Type I to Skin Type III. Emsculpt Neo® is also cleared for improvement of abdominal tone, strengthening of the abdominal muscles and development of firmer abdomen. Strengthening, toning, firming of buttocks, thighs, and calves. Improvement of muscle tone and firmness, for strengthening muscles in arms. 1Abdominal toning and reduction of subcutaneous fat with combination of novel radiofrequency treatment and HIFEM procedure – MRI scan study. Jacob C. et al. Presented at ASDS 2020 Virtual Meeting. 2Radiofrequency heating and HIFEM delivered simultaneously – the first sham-controlled randomized trial. Katz B. et al. Presented at ASDS 2020 Virtual Meeting. 3Ultrasound evaluation of the simultaneous RF and HIFEM treatments on human fat tissue. Denkova R. Source: U.S. Food and Drug Administration. 510(k) Premarket Notification: K192224. Published online on December 5, 2019. 4Halaas Y, Duncan D, Bernardy J, Ondrackova P, Dinev I. Activation of Skeletal Muscle Satellite Cells by a Device Simultaneously Applying High-Intensity Focused Electromagnetic Technology and Novel RF Technology: Fluorescent Microscopy Facilitated Detection of NCAM/CD56. Aesthet Surg J. 2021.
Emsculpt® is intended for improvement of abdominal tone, strengthening of the abdominal muscles, development of firmer abdomen. Strengthening, toning and firming of buttocks, thighs, and calves. Improvement of muscle tone and firmness, for strengthening muscles in arms.