BTL Emtone
BTL Emtone
Emtone works by using a combination of heat via radiofrequency (RF) energy and targeted pressure to hit the primary sources of cellulite. What you’re left with is a boost in new collagen and improved elastin, two key components that will give you the dimple-free skin that can be so elusive. Rock those shorts, smooth your tummy and enhance the skin’s laxity in any areas suffering from cellulite.
Take that next step to smooth and tighten your skin with Emtone in Orange County. Our experts at the Med Lounge can help you come up with an individualized cellulite treatment plan that will get your skin on its way to its new, dimple-free existence. Love the skin you’re in again with Emtone.
Benefits of Emtone
- Smooth and tighten
- Non-invasive treatment
- Zero downtime
- Improve skin laxity
- Boost collagen production
- Zero downtime
- Confidence boost
- Painless and effective
- Works for all skin types
- Extremely safe
FREQUENTLY ASKED QUESTIONS
Yes, Emtone reduces cellulite. It does so by heading off the main reasons why cellulite happens in the first place: reduced skin elasticity, enlarged fat chambers under the skin, poor blood flow, waste and water retention, and rigid fibers beneath the skin that cause that tell-tale dimpling.
Repeated treatments as part of an individualized Emtone treatment plan lead to not only an overall reduction of cellulite over time but improved skin laxity and texture within weeks of your treatments.
Emtone works by combining dual technologies (thermal and mechanical energy) to target the causes of cellulite. Thermal energy comes in radiofrequency (RF) energy, heating the skin to tighten the skin’s fibers and boost collagen production.
Mechanical energy in the form of acoustic energy penetrates the skin, boosting circulation and reducing waste and fluid retention that is another culprit behind cellulite.
Both processes disrupt the fat chambers and rigid skin fibers, creating those uneven, dimpled surfaces in the upper layers of the skin. Emtone uses your skin’s natural processes to stimulate a response that supports new collagen and elasticity, dramatically improving the appearance of cellulite in targeted areas.
Emtone doesn’t hurt. Some patients even compare it to a hot stone massage with mechanical vibrations as your provider applies the device to your skin. The heating effect comes from the radiofrequency energy used as part of the treatment. At the same time, your provider uses targeted pressure energy to boost circulation in your treatment zones. All of this happens while you’re lying down and relaxing for the 20 minutes or so your treatment will take.
There’s no downtime after your treatment, so you’ll be able to get back to your day-to-day activities right after your therapy.
Your Emtone results will last anywhere from three months to a year. While some will see improvements to their cellulite after just one session, don’t expect final results until about three months after your last session. The wait is worth it!
Touchup treatment sessions are recommended to maintain those skin-smoothing results. How often you come in for a touchup will depend on your body. Some come in annually to see the results they crave, while others will need more frequent visits to maintain results.
See the amazing difference in our clients, before and after treatment.
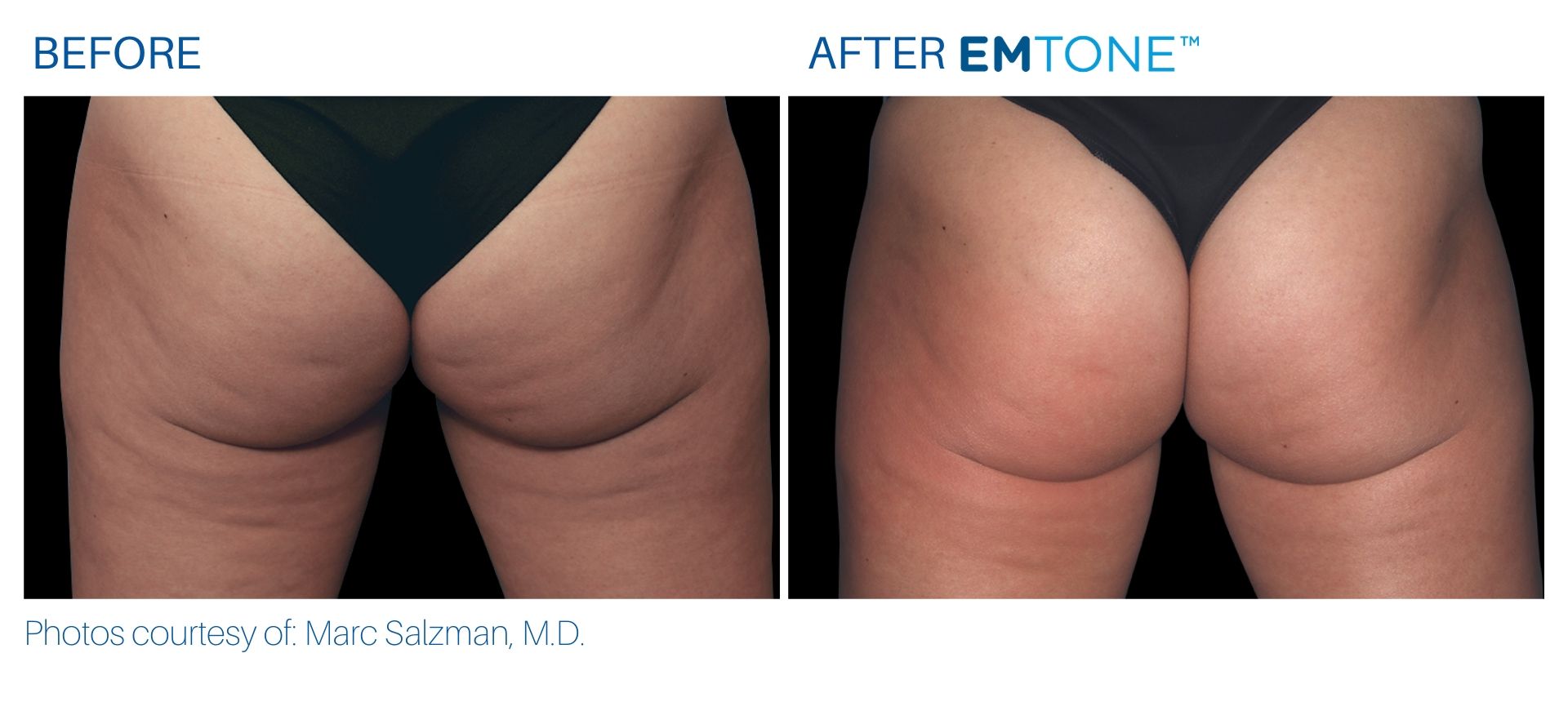
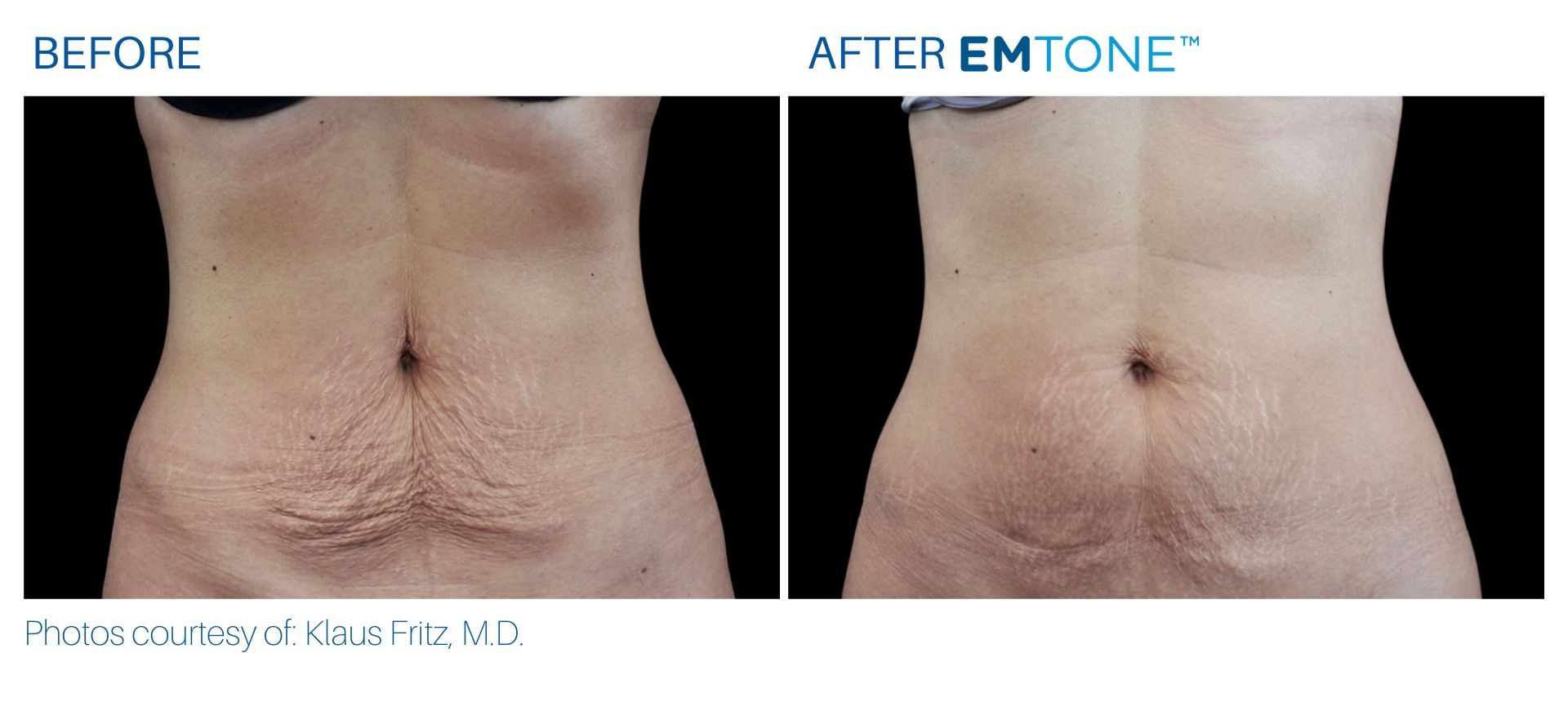
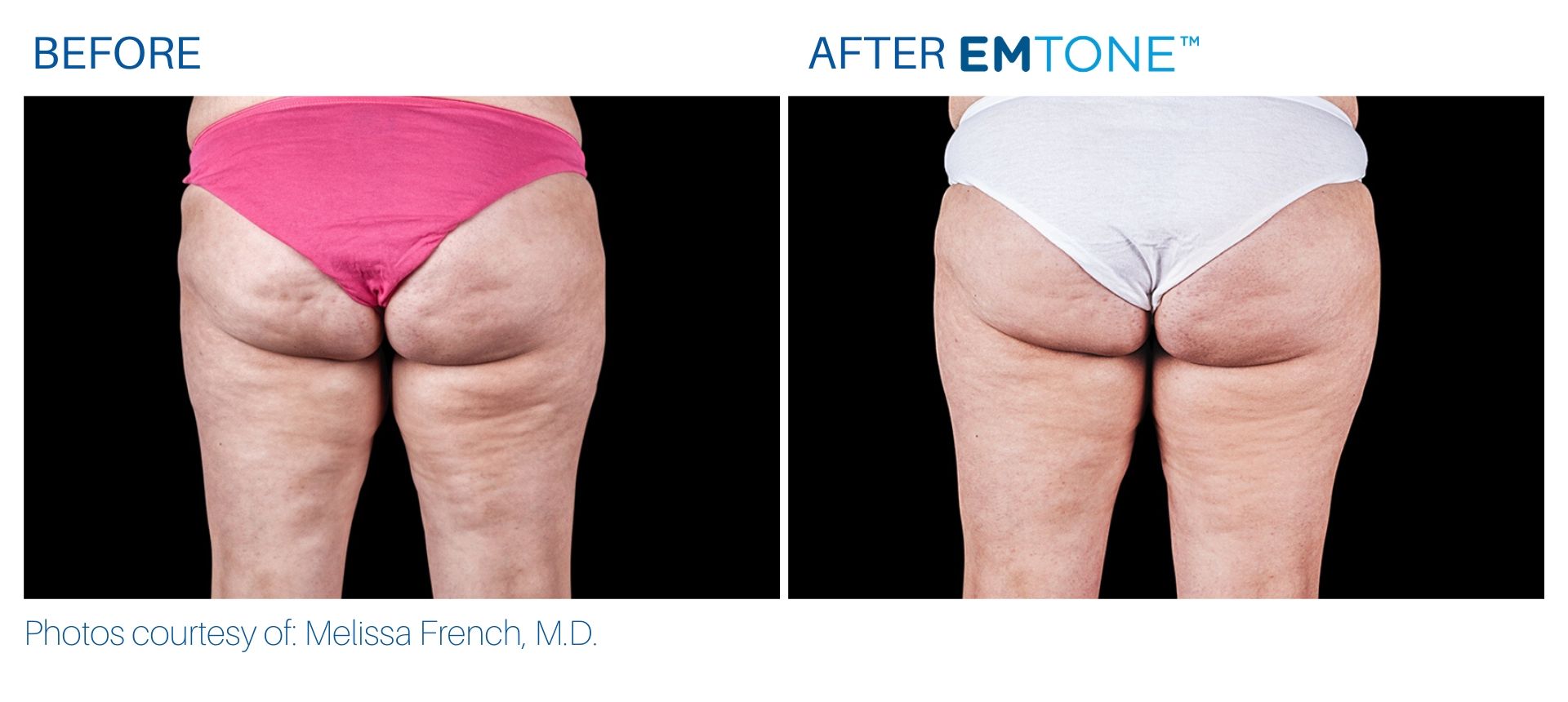
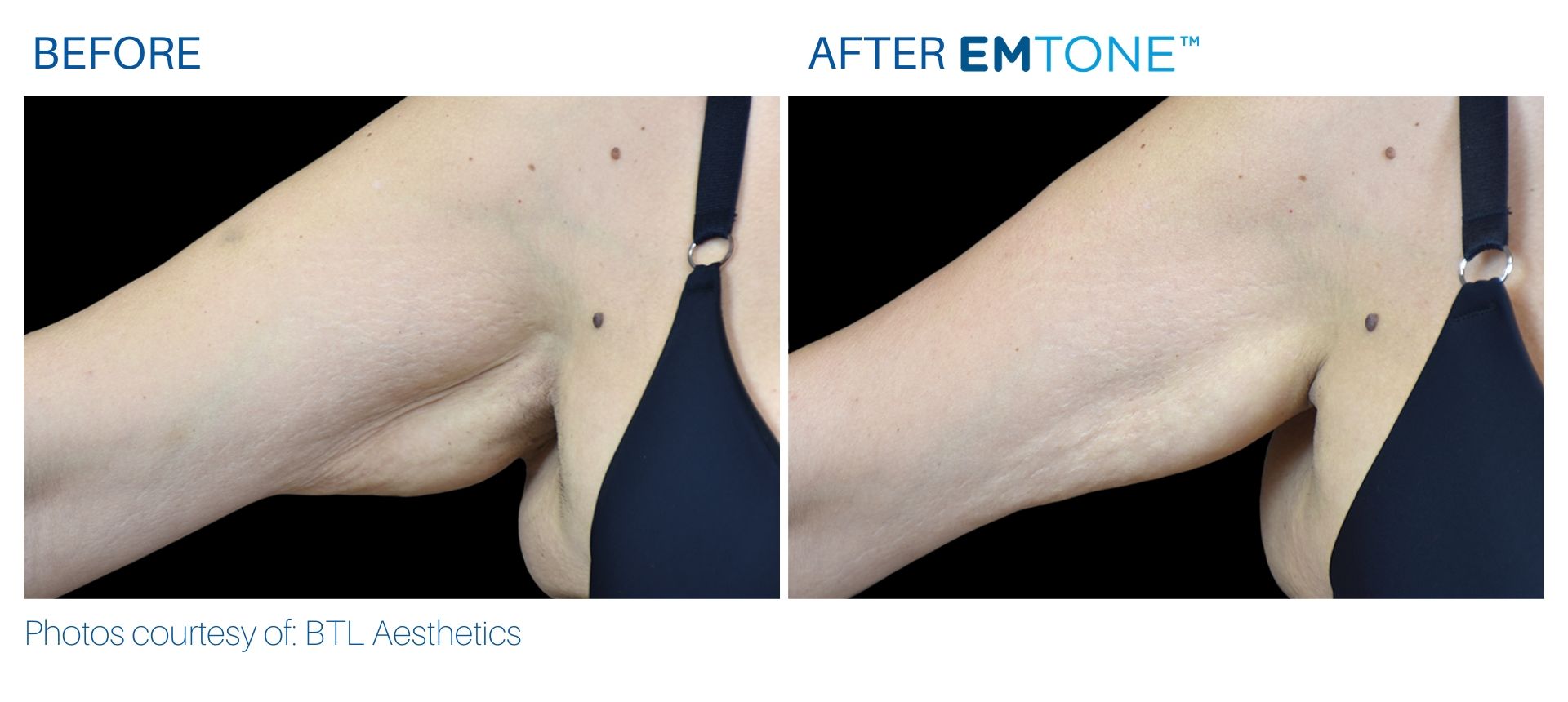
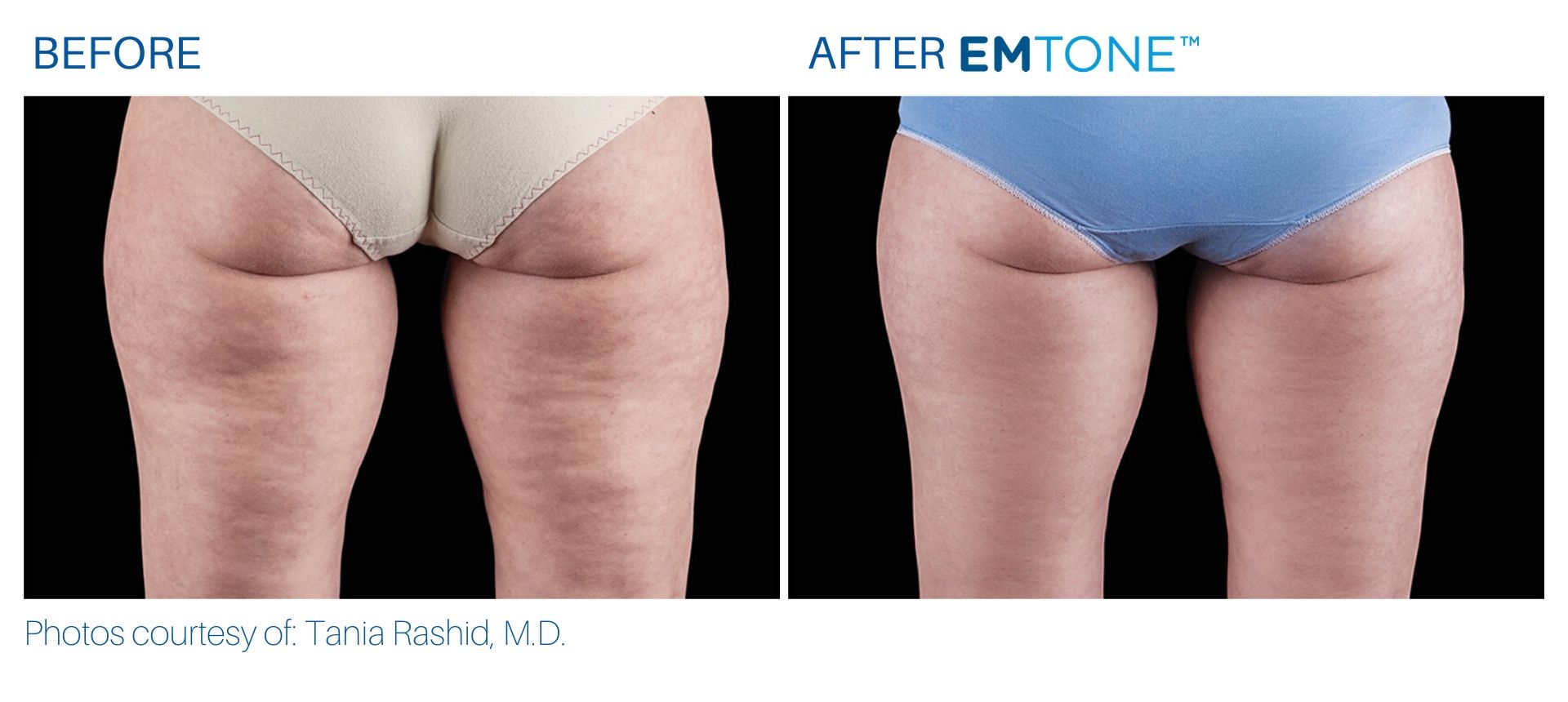
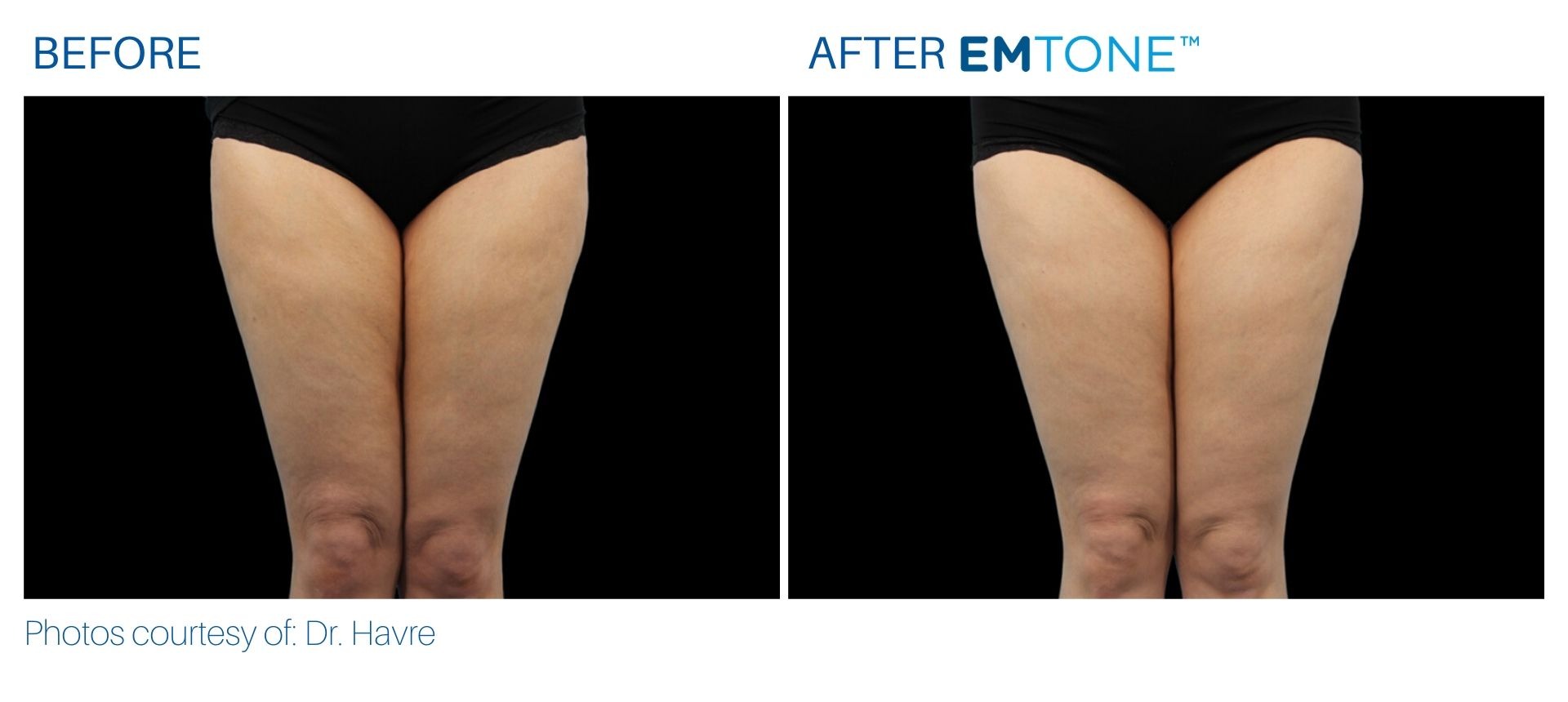
What to Expect With Emtone
PRE-TREATMENT
- Wear comfortable clothing on the day of your treatment. Your provider will need easy access to areas being targeted during your procedure.
- Leave the skin free of any lotions, makeup or serums the day of your treatment.
- Remove any jewelry and put away electronic devices before starting your treatment.
- Hydration is important. Come to your appointment well-hydrated, and drink plenty of water in the days leading up to your treatment. Avoid beverages with a diuretic effect, like drinks containing caffeine and alcohol, in a day or two ahead of your treatment.
- Talk to your provider about any cardiovascular conditions, implants or injuries you’ve experienced in the areas you’d like to target. Your provider will be able to tell you whether Emtone is safe for you or whether you’re a better candidate for a different kind of treatment that will get you the results you’re looking for.
POST-TREATMENT
- You shouldn’t expect any downtime after treatment and should be able to return to your regular, daily activities right after your treatment.
- You may experience some soreness in the treated area in the days post-treatment. This is normal and will go away on its own within a day or so.
- Any redness you see on your skin after your treatment is temporary and should go away the same day of your Emtone procedure. This is a result of the heating effects of your Emtone treatment.
- Keep well-hydrated in the days after treatment. Hydration boosts elasticity in the skin, improves tone, and helps you feel your best after your procedure.
- The recommended minimum number of sessions for optimal results is four sessions. Some patients will benefit from six total sessions, depending on the treatment areas targeted. Schedule your sessions as recommended by your provider at the end of your Emtone appointment, and follow treatment instructions to the letter.