KYBELLA INJECTIONS
Kybella Injections
Kybella is a targeted fat cell destroyer that tackles that age-old problem of getting rid of chin fat. It’s most popular for dissolving submental fullness or the area beneath your chin that causes saggy jowls and that dreaded double chin. The key to Kybella is in its formula. It’s a synthetic, human-made form of deoxycholic acid, naturally occurring bile that aids in the breakdown of fatty tissue in the body.
Benefits of Kybella Injections
- Dissolves fat for good
- Tightens skin
- Natural results
- Improvement over time
- Long-lasting results
- Quick procedure
- Minimally-invasive
- Quick recovery
- Targets problem areas
- Extremely safe
FREQUENTLY ASKED QUESTIONS
The injectable used in Kybella is a synthetic form of deoxycholic acid, a naturally-occurring bile acid in the body that aids in the breakdown of fatty tissue. When injected into the submental area of the chin or other targeted problem areas, it destroys the fall cells in those areas for good.
Over time and over a number treatments — your provider will likely recommend between 2-4 for optimal results — the injectable keeps those targeted areas from storing or accumulating fat. All that you’re left with is your desired result.
Kybella is ideal for patients looking to target stubborn pockets of fat where traditional methods haven’t been successful. The double chin is the most popular area to treat, but there are other problem spots that have been proven Kybella-worthy:
- Bra bulge
- Love handles
- Knee fat
- Saddlebags
- Lower abdomen
Former liposuction or C-section patients have also turned to Kybella to reduce bulges or pockets of fat as a result of those surgeries. Kybella is also a wonder for those close to their personal goal weight, especially if they’re having difficulties targeting fat loss in problem areas.
If you’re looking to treat larger areas, experts at the Med Lounge can help you decide on nonsurgical alternatives that may better meet your needs over Kybella. If you’re pregnant, may become pregnant or are currently breastfeeding, you should not be receiving Kybella injections.
Before your treatment session, your physician will mark where your injections will go. They will then numb the area to weaken any potential discomfort. Some patients report a burning sensation during their treatment. The treatment itself will take around 15 minutes from start to finish.
Treatments will be scheduled every 4-6 weeks, depending on your injection sites and personalized treatment plan. That treatment plan will determine your Kybella cost. Expect 2-4 treatments for optimal results.
Kybella is very safe. Synthetic deoxycholic acid mimics naturally-produced acids in your body, reducing the chance for reactions post-treatment. Kybella is FDA-approved for use in targeted fat cell reduction in the chin area and has been used globally for years in destroying fat cells in problem spots.
Some swelling is expected post-treatment, but that subsides over time. Pain and redness can usually be managed with over-the-counter pain medications and ice packs to the injection site.
Kybella dissolves fat cells at the injection sites, so results are meant to be long-lasting. Those fat cells don’t come back. They’re destroyed in the process. Once you reach your desired results with Kybella, it’s not expected that you’ll need to return for additional treatments.
See the amazing difference in our clients, before and after treatment.

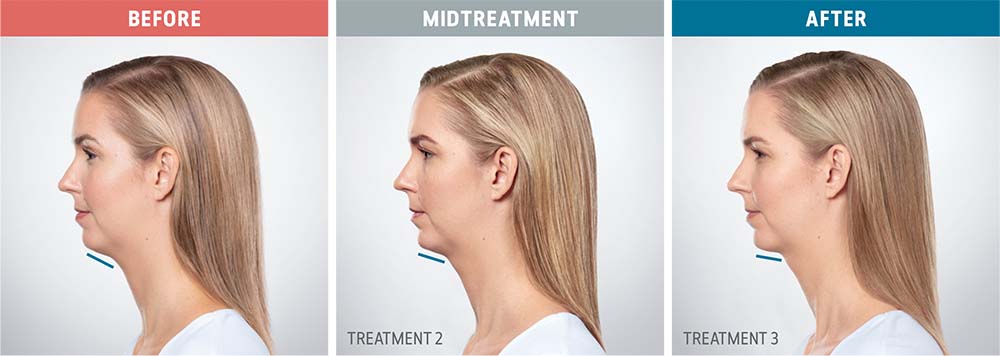
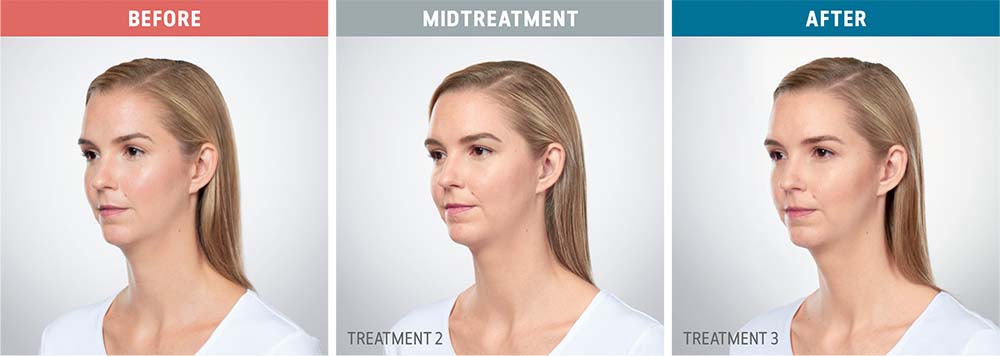

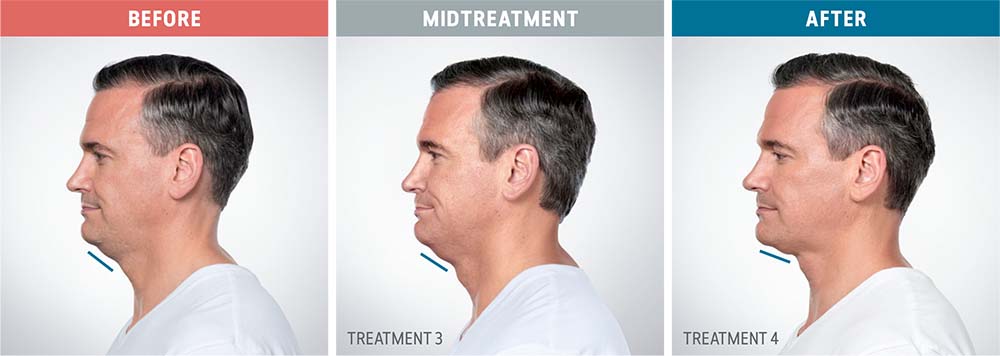

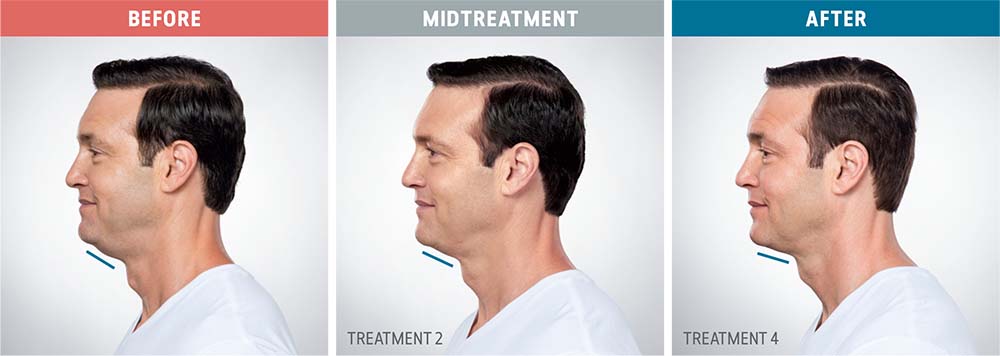


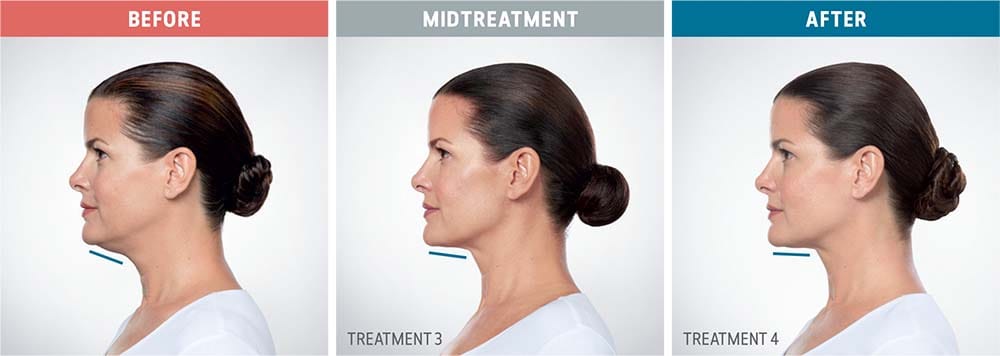

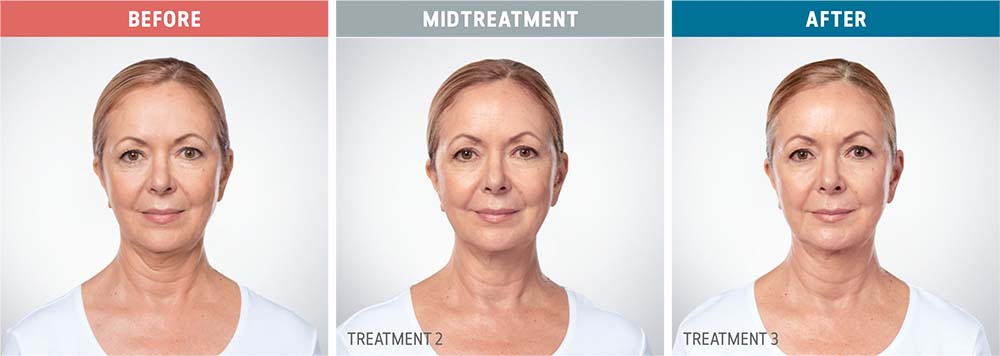
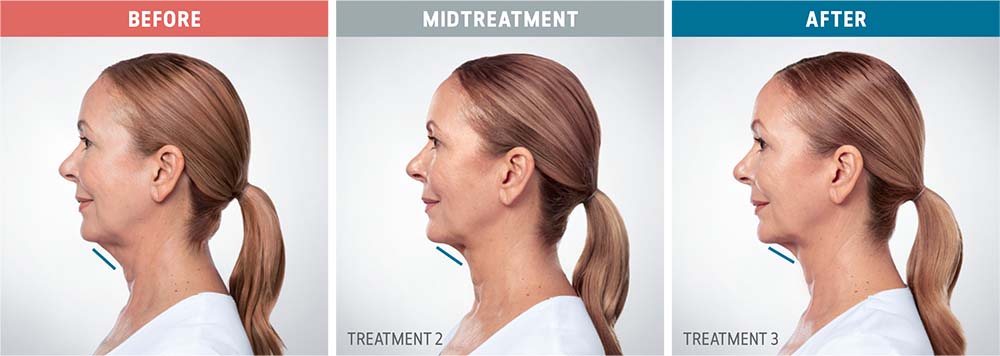
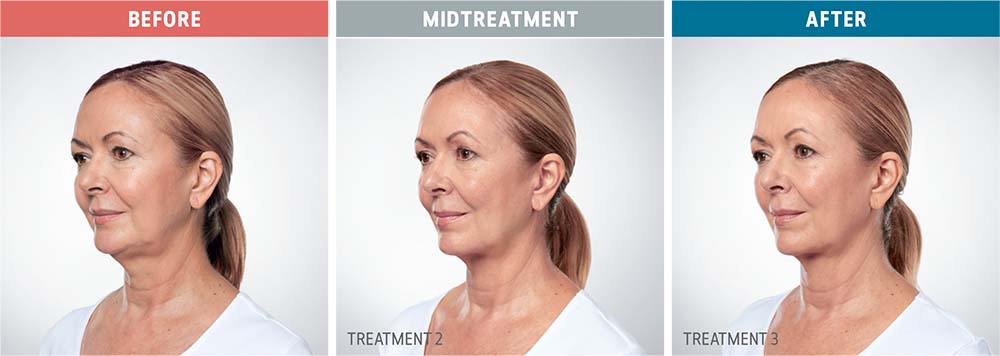
What to Expect With Kybella
PRE-TREATMENT
- Avoid any anti-inflammatories or blood-thinning medications up to two weeks before your treatment. Those include but aren’t limited to: aspirin, Motrin, ibuprofen, and Aleve. If you take daily aspirin for a history of heart disease, talk to your doctor before stopping your aspirin routine.
- Try to avoid scheduling any special events in the two weeks after your Kybella injections. Some swelling in the targeted areas is expected. It’s natural and temporary.
- Avoid caffeine, alcohol and high sodium foods at least 48 hours before and 48 hours after your treatment to avoid increased swelling or irritation at the injection sites.
- Come to your appointment well-hydrated. Drink plenty of water in the days leading up to your Kybella injections.
- Consider taking Arnica tablets several days ahead of your treatment. Arnica could help prevent swelling at your injection sites.
- Come to your appointment fresh-faced if you’re receiving injections in and around the face. That means no lotions, creams or makeup at the treatment sites.
- Talk to your provider if you’ve had any cosmetic treatments within the last 14 days before your Kybella treatment. Share a complete medical history, particularly if you’ve had surgeries on your chin, jaw or neck.
POST-TREATMENT
- Avoid rubbing or massaging your face the day after your Kybella treatment.
- Keep yourself hydrated by drinking plenty of water in the days following your treatment.
- To reduce swelling, apply a cold compress or ice pack to the targeted areas for 20 minutes at a time every hour in the 24 hours after your treatment.
- If you’re feeling some discomfort, take an over-the-counter painkiller or acetaminophen to help you feel better.
- Avoid strenuous exercise and intense sun exposure for up to 5 days after your treatment. Rest and relax instead. If you’ve planned any naps, it’s recommended that you sleep on your back with your head elevated for at least three days after treatment.
- If it’s your first round of injections, your provider will schedule a series of Kybella injections for maximum benefit. Stick to the schedule your provider shares with you for ideal results.
- Call your doctor if you have any serious side effects, such as increased pain or swelling, muscle weakness or difficulty swallowing. Some swelling at the injection site is all normal and temporary.
Safety Information
KYBELLA® (deoxycholic acid) injection 10 mg/mL Important Information
INDICATION
KYBELLA® (deoxycholic acid) injection is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults.
The safe and effective use of KYBELLA® for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
KYBELLA® is contraindicated in the presence of infection at the injection sites.
WARNINGS AND PRECAUTIONS
Marginal Mandibular Nerve Injury
Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness, were reported in 4% of subjects in the clinical trials; all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA® should not be injected into or in close proximity to the marginal mandibular branch of the facial nerve.
Dysphagia
Dysphagia occurred in 2% of subjects in the clinical trials in the setting of administration-site reactions, eg, pain, swelling, and induration of the submental area; all cases of dysphagia resolved spontaneously (range 1-81 days, median 3 days). Avoid use of KYBELLA® in patients with current or prior history of dysphagia as treatment may exacerbate the condition.
Injection-Site Hematoma/Bruising
In clinical trials, 72% of subjects treated with KYBELLA® experienced hematoma/bruising. KYBELLA® should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the treatment area may occur.
Risk of Injecting into or in Proximity to Vulnerable Anatomic Structures
To avoid the potential of tissue damage, KYBELLA® should not be injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph nodes, and muscles. Care should be taken to avoid inadvertent injection directly into an artery or a vein as it can result in vascular injury
Injection Site Alopecia
Cases of injection site alopecia have been reported with administration of KYBELLA®. Onset and duration may vary among individuals and may persist. Consider withholding subsequent treatments until resolution.
Injection Site Ulceration and Necrosis
Injections that are too superficial into the dermis may result in skin ulceration and necrosis. Cases of injection site ulceration and necrosis have been reported with administration of KYBELLA®. Do not administer KYBELLA® into affected area until complete resolution.
ADVERSE REACTIONS
The most commonly reported adverse reactions in the pivotal clinical trials were: injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and induration.
Please see KYBELLA® full Prescribing Information.